Indication: Matulane is indicated for use in combination with other anticancer drugs for the treatment of Stage III and IV Hodgkin’s disease. Matulane is used as part of the MOPP (nitrogen mustard, vincristine, procarbazine, prednisone) regimen.
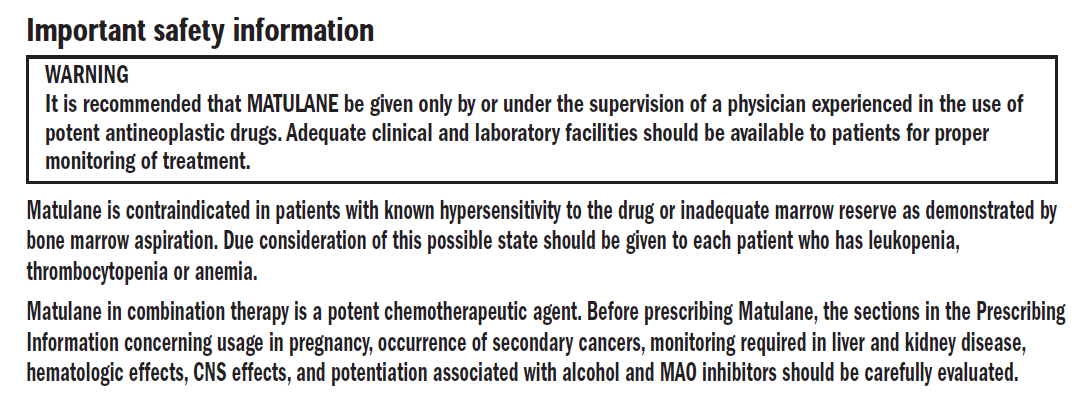
Please see full Prescribing Information for Matulane indications and usage, contraindications, and warnings including boxed WARNINGS, precautions, and adverse reactions.
Click here for information to how to obtain the product.